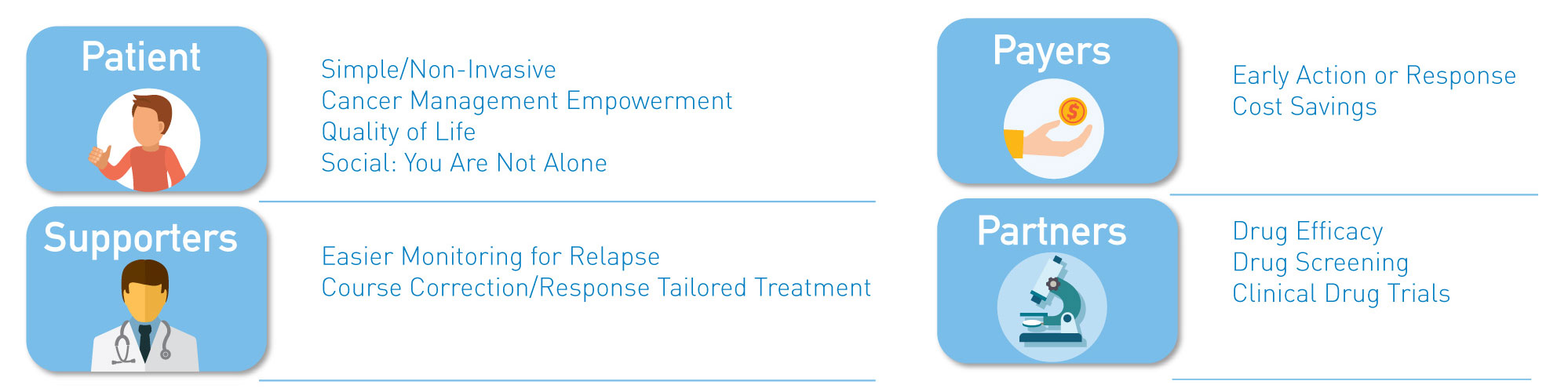
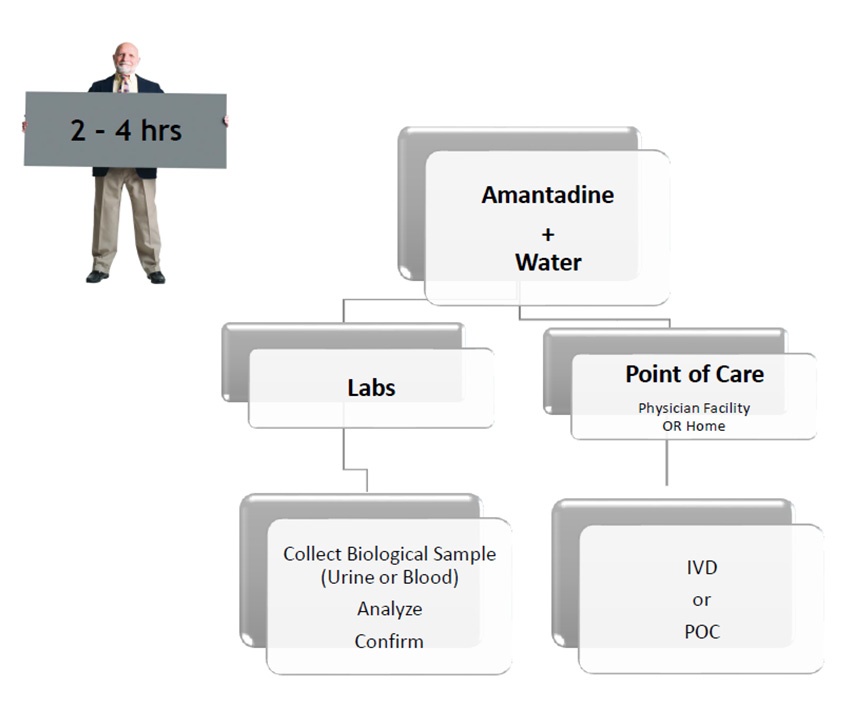
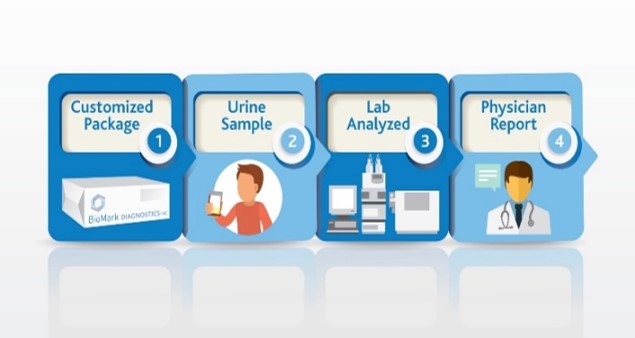
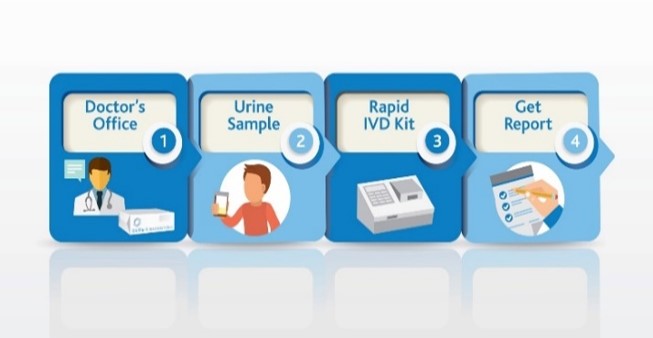
Use SSAT1 Red Alert – General low-cost test for high risk individuals during routine test in cancers that have been associated with high levels of this enzyme. Lung, breast, prostate, thyroid and glioblastoma. The same assay can be utilized to measure response following treatment and to monitor for recurrence once baselines are established in the cancer survivor population group
Lung Cancer Metabolite Panel – Early detection of lung cancer in high risk population and to complement Low dose CT screening for lung cancer.
Below is an abstract* that provides a potential market impact of our diagnostic test success for this application. (BioMark’s assay advantages include low cost, minimally invasive, low false positives and leverages existing lab infrastructure)
* Abstract:
Source: January 2017 Volume 18, Issue 1, Pages e27–e34 Clinical Lung Cancer
Purpose
To assess the diagnostic costs leading up to a lung cancer diagnosis in patients with abnormal computed tomography (CT) scans.
Patients and Methods
A retrospective cohort study using the 5% Medicare claims data (January 1, 2009, to December 31, 2011) was conducted. Patients aged 65 to 74 years with an abnormal chest CT scan were identified. Index was defined as the date of the abnormal chest CT scan. Outcomes assessed over a 12-month follow-up after index included lung cancer diagnosis rate and the use and associated costs of follow-up diagnostic tests up to diagnosis of lung cancer.
Results
Of 8979 patients identified with an abnormal chest CT scan (mean age, 69.3 ± 2.9 years), 13.9% were diagnosed with lung cancer over 12 months. Chest x-rays were the most common diagnostic test. Of the 19% who underwent a biopsy, 43.6% were not diagnosed with lung cancer during follow-up. The average total diagnostic assessment cost per patient was higher for those with versus without lung cancer ($7567 vs. $3558). Among patients not diagnosed with lung cancer, the median diagnostic cost per patient for those with versus without biopsy was ∼ 28 times higher. Adverse events significantly increased the average cost per biopsy (approximately 4-fold).
Conclusion
Total lung cancer diagnostic cost was $38.3M in the defined study sample, of which 43.1% was accounted for by biopsied patients without a lung cancer diagnosis. Additional risk stratification is required to decrease unnecessary biopsy referrals and costs. Further, adverse events significantly increased costs.
Keywords:
5% Medicare, Adverse events, Biopsy, Diagnosis, Retrospective cohort