BIOMARK TO OPEN A DIAGNOSTIC LABORATORY IN QUÉBEC

Vancouver, British Columbia – (April 29, 2021) – BioMark Diagnostics Inc. (“BioMark”) (CSE: BUX) (FSE: 20B) (OTCMKTS: BMKDF) is pleased to announce today that its wholly-owned subsidiary BioMark Diagnostic Solutions Inc. (“BDS”) will be operating a diagnostic laboratory in Quebec, Canada, which will primarily serve to advance the clinical validation of its proprietary liquid biopsy […]
BIOMARK ANNOUNCES EXERCISE OF WARRANTS AND GAIN DTC ELIGIBILITY FOR ITS COMMON SHARES

Vancouver, British Columbia – (April 21, 2021) – BioMark Diagnostics Inc. (“BioMark”) (CSE: BUX) (FSE: 20B) (OTCMKTS: BMKDF) is pleased to announce today the agreement by several long-time strategic accredited investors and insiders to exercise approximately 2,000,000 warrants issued in connection with the Company’s private placement of units completed in April 2019, with the warrant […]
BIOMARK’S COLLABORATOR RECEIVES FUNDING TO VALIDATE ITS BIOMARKER PANEL FOR THE EARLY DETECTION OF LUNG CANCER
Vancouver, British Columbia – (March 16, 2021) – BioMark Diagnostics Inc. (“BioMark”) (CSE: BUX) (FSE: 20B) (OTCMKTS: BMKDF) is pleased to announce today that its sponsored research collaboration with The Metabolomics Innovation Centre (TMIC) was successful in receiving funding from the Novel Technology Application in Cancer Prevention and Early Detection Spark Grants competition. This comes […]
BIOMARK GRANTS OPTIONS

Vancouver, British Columbia – (March 2, 2021) – BioMark Diagnostics Inc. (“BioMark”) (CSE: BUX) (FSE: 20B) (OTCMKTS: BMKDF) is pleased to announce that it has granted 2,100,000 incentive stock options under the Company’s Stock Option Plan (“Option Plan”) to third-party consultants to support market communication and corporate strategy as it advances its commercialization efforts. Each […]
BIOMARK AND PHYTRONIX TECHNOLOGIES INC. ENTER INTO A COLLABORATIVE RESEARCH AND DEVELOPMENT AGREEMENT
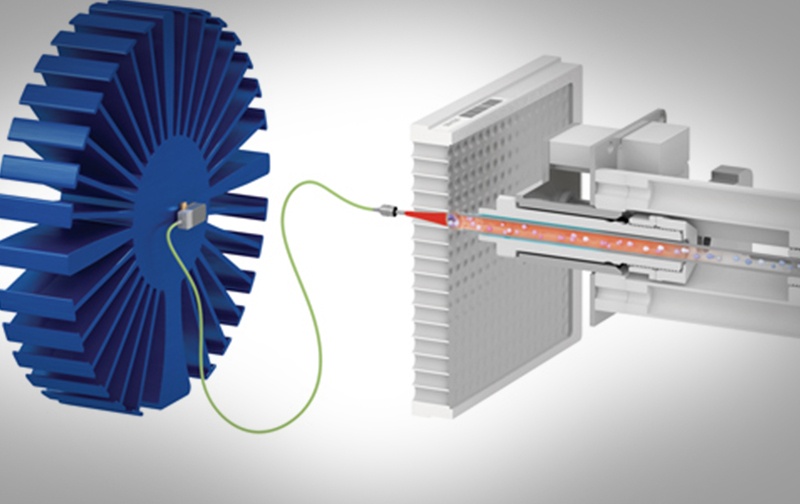
Vancouver, British Columbia – (February 16, 2021) – BioMark Diagnostics Inc. (“BioMark”) (CSE: BUX) (FSE: 20B) (OTCMKTS: BMKDF) is pleased to announce that its wholly-owned subsidiary BioMark Diagnostic Solutions Inc. has entered into a collaborative research and development agreement with Phytronix Technologies Inc. (“Phytronix”) to advance the development of BioMark’s early lung cancer screening applications […]
BIOMARK DIAGNOSTICS TARGETS RESPONSE TO TREATMENT APPLICATION WITH ITS LIQUID BIOPSY PLATFORM

Vancouver, British Columbia – (February 8, 2021) – BioMark Diagnostics Inc. (“BioMark”) (CSE: BUX) (FSE: 20B) (OTCMKTS: BMKDF) is pleased to announce that the article “Use of Amantadine in the Evaluation of Response to Chemotherapy in Lung Cancer – a Pilot Study” has been published in the peer-reviewed journal Future Science OA. Rashid A. Bux, […]
BIOMARK RETAINS QUESTRADE, INC. TO PROVIDE MARKET MAKING SERVICES

Vancouver, British Columbia – (December 7th, 2020) – BioMark Diagnostics Inc. (“BioMark”) (CSE: BUX) (FSE: 20B) (OTCMKTS: BMKDF) announced today that it has retained Questrade, Inc. (“Questrade”) to provide market-making services in accordance with CSE guidelines. The term of the agreement is for one year, beginning December 7, 2020 at a cost of CAD $3,000 per month. Either […]
Alfred Berkeley Joins BioMark’s Advisory Team

Vancouver, British Columbia – (January 11, 2021) – BioMark Diagnostics Inc. (“BioMark”) (CSE: BUX, FSE: 20B, OTCMKTS: BMKDF) is glad to announce Alfred Berkeley as a new member of the BioMark’s Advisory team as he will be providing strategic and financial advice to help expand the company’s commercialization effort in the U.S. Mr. Rashid Ahmed […]
BIOMARK SCIENTIFIC ADVISOR DR. DONALD MILLER TO PRESENT AT THE DELAWARE VALLEY DRUG METABOLISM DISCUSSION GROUP

Vancouver, British Columbia – (September 23rd, 2020) – BioMark Diagnostics Inc. (“BioMark”) (CSE: BUX) (FSE: 20B) (OTCMKTS: BMKDF) is pleased to announce that on September 29, its strategic scientific advisor Dr. Donald Miller is to present on BioMark’s SSAT amantadine assay for the early detection of cancer at the prestigious Delaware Valley Drug Metabolism Discussion […]
BIOMARK APPOINTS DR. JEAN-FRANCOIS HAINCE AS STRATEGIC ADVISOR TO GUIDE ITS EXPANSION INTO QUEBEC

Vancouver, British Columbia – (September 16th, 2020) – BioMark Diagnostics Inc. (“BioMark”) (CSE: BUX) (FSE: 20B) (OTCMKTS: BMKDF) is pleased to announce that it has appointed Dr. Jean-Francois (Jeff) Haince as a strategic advisor to its management team. Rashid Ahmed, President and CEO, says, “We are delighted to welcome Dr. Haince into our advisory team. […]
